H-atom p.9
Aufbau: Building Up Multi-Electron Atoms
A consequence of the Spin-Statistics Theorem is the
Pauli Exclusion Principle which states
that at most two electrons can be in the same spatial wavefunction.
(If two electrons share a spatial wavefunction, they must be in the
spin "paired", i.e., singlet, state.)
The lessons learned from the Helium atom are that
- as a result of the electron-electron repulsion the n2
states (ns, np, up to l=n-1) that were
degenerate in the H-atom are instead "l-tilted" so the
ns level lies below the np level, etc.
- other things being equal, the spatially antisymmetric state
(and hence the state with "all the electron spinning the same way")
has lowest energy.
Follow along as we build up the atoms of the period table. (Denoted
below is the electron configuration followed by
[2S+1LJ] which describes the
ground state term.
- H: 1s (one electron in the 1s state)
[2S1/2]
- He: 1s2 (two electrons in the 1s state, that state is now full)
[1S0]
That completes the first row
- Li: 1s22s (two electrons filling the 1s state, because
of l-tilting the next available state is 2s)
[2S1/2]
- Be: 1s22s2 (two electrons filling the 1s state;
two electrons filling the 2s state)
[1S0]
- B: 1s22s22p (two electrons filling the 1s state;
two electrons filling the 2s state; the last electron in the lowest available state: 2p)
[2P1/2]
- C: 1s22s22p2
[3P0]
- N: 1s22s22p3
[4S3/2]
- O: 1s22s22p4
[3P2]
- F: 1s22s22p5
[2P3/2]
- Ne: 1s22s22p6
[1S0]
That completes the second row
- Na: 1s22s22p63s
[2S1/2]
- Mg: 1s22s22p63s2
[1S0]
- Al: 1s22s22p63s23p
[2P1/2]
- Si: 1s22s22p63s23p2
[3P0]
- P: 1s22s22p63s23p3
[4S3/2]
- S: 1s22s22p63s23p4
[3P2]
- Cl: 1s22s22p63s23p5
[2P3/2]
- Ar: 1s22s22p63s23p6
[1S0]
Surprisingly that completes the third row. l-tilting is so extreme that the 4s has slightly
lower energy than 3d. For larger Z the 3d will go below 4s, but currently
4s fills.
- K: 1s22s22p63s23p64s
[2S1/2]
- Ca: 1s22s22p63s23p64s2
[1S0]
Now we start to fill 3d in row 4! The result is the "transition elements". There are two oddities
it the filling sequence...can you find them? I'm tired of repeating all the lower levels...I'll just
use: (Ar) to mean the filled shells of Argon: 1s22s22p63s23p6
- Sc: (Ar) 3d4s2
[2D3/2]
- Ti: (Ar) 3d24s2
[3F2]
- V: (Ar) 3d34s2
[4F3/2]
- Cr:(Ar) 3d54s
[7S3]
- Mn: (Ar) 3d54s2
[6S5/2]
- Fe: (Ar) 3d64s2
[5D4]
- Co: (Ar) 3d74s2
[9F9/2]
- Ni: (Ar) 3d84s2
[3F4]
- Cu: (Ar) 3d104s
[2S1/2]
- Zn: (Ar) 3d104s2
[1S0]
The below is a physicist's view of the periodic table. Helium has been moved over from its
proper place with the other inert gases on the far right, to above the "alkaline earth" elements like
Magnesium. Thats to make the normal filling sequence more systematic: you just
move across the row. Note the insertion of the "rare earth elements"
the "lanthanides" and "actinides"; in rows n=6,7 we start filling
the (n-2)f orbitals.
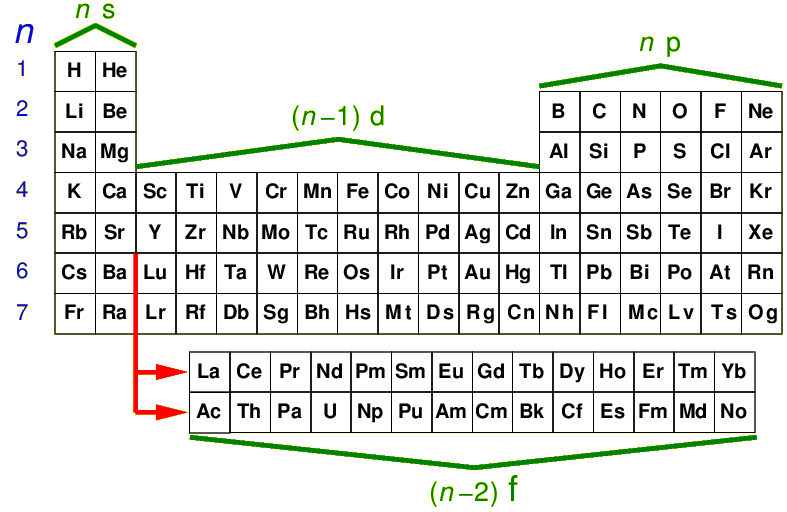
As you might expect for larger n the levels are more closely
spaced (just as in the H-atom), and unexpected fillings become more common.
Here are some "odd" configurations:
- Pd: (Kr)4d10
[1S0]
- La: (Xe)5d6s2
[2D3/2]
- Ce: (Xe)4f5d6s2
[1G4]
- Gd: (Xe)4f75d6s2
[9D2]
- Pt: (Xe)4f145d96s
[3D3]
- Th: (Rn)6d27s2
[3F2]
- U: (Rn)5f36d7s2
[5L6]
- Lr: (Rn)5f147s27p
[2P1/2]
Next

