Phases and Phase Equilibria
Keywords: solid, liquid, gas, kinetic molecular theory, phase diagram
- Gas Phase
- Standard temperature and pressure; standard molar volume
- Ideal Gas
- Ideal gas law (PV = nRT)
- Boyle's law
- Charles' law
- Kinetic molecular theory of gases
- Qualitative aspects of deviation of real gas behavior from ideal gas law
- Partial pressure, mole fraction
- Dalton's law relating partial pressure to composition
- Liquid phase (inter- and intra-molecular forces)
- Hydrogen bonding
- Dipole interaction
- Van def Waals' forces
- Phase equilibria (solids, liquids, gases)
- Phase changes and phase diagrams
- Freezing point, melting point, boiling point
- Molality
- Colligative properties
- Vapor-pressure lowering (Raoult's law)
- Boiling-point elevation
(
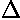 Tb = Kb m)
Tb = Kb m)
- Freezing-point depression
(
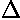 Tf = Kf m)
Tf = Kf m)
- Osmotic pressure
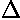 Tb = Kb m)
Tb = Kb m)
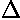 Tf = Kf m)
Tf = Kf m)