Thermodynamics and Thermochemistry
Keywords: endo/exo-thermic, enthalpy, calorimetry, thermodynamics
- Thermochemistry
- Thermodynamic system, state function
- Conservation of energy
- Endothermic/exothermic reactions
- Enthalpy
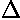 H and standard heats
of reaction
H and standard heats
of reaction
- Hess' law of heat summation
- Bond dissociation energy as related to heats of reaction
- Measurement of heat changes (calorimetry)
- Heat capacity
- Specific heat (e.g., cwater approx 1 cal/g·°C)
- Entropy as a measure of "disorder", relative entropy for gas, liquid, and crystal
states
- Gibbs free energy G
- Spontaneous reactions and
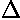 G0
G0
- Thermodynamics
- First law:
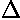 E = Q - W
E = Q - W
- Equivalence of mechanical, chemical, and thermal energy units
- Temperature scales
- Heat transfer
- Conduction
- Convection
- Radiation
- Coefficient of expansion
- Heats of fusion, vaporization
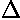 H and standard heats
of reaction
H and standard heats
of reaction
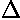 G0
G0
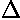 E = Q - W
E = Q - W