Rate Processes in Chemical Reactions: Kinetics and Equilibrium
Keywords:rate constants, equilibrium constants, catalysts
- Reaction rate
- Dependence of reaction rate upon concentrations of reactants
- Rate law
- Rate constant
- Reaction order
- Rate-determining step
- Dependence of reaction rate upon temperature; activation energy
- Activation complex or transition state
- The interpretation of energy profiles showing energies of reactants
and products, activation energy, and
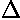 H
for the reaction
H
for the reaction
- Kinetic control vs. thermodynamic control of a reaction
- Catalysts; the special of enzyme catalysis
- Equilibrium in reversible chemical reactions
- Law of mass action
- The equilibrium constant
- Application of LeChatelier's principle
- Relationship of the equilibrium constant and
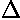 G0
G0
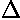 H
for the reaction
H
for the reaction
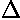 G0
G0